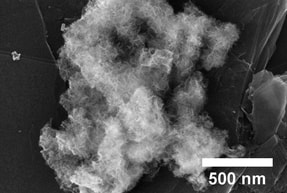
Energy and environment are two of our critical societal challenges. The use of hybrid nanomaterials to harvest solar energy as well as capture and convert CO2 seems to be the best way combat climate change. We recently reported the synthesis of a new class of dendritic fibrous nano-silica (DFNS). Fibrous morphology observed in these nanospheres has not been seen before in silica materials. Uniqueness of DFNS is, its high surface area is by virtue of its fibrous structure instead of pores (unlike MCM-41 and SBA-15 silicas), and hence easily accessible. More than 100 groups worldwide is now using our patented DFNS for various applications such as catalysis, solar-energy harvesting, energy storage, self-cleaning antireflective coatings, surface plasmon resonance-based ultrasensitive sensors, CO2 capture, and biomedical applications.1d We showed successful utilization of DFNS for range of important catalytic applications such as metathesis, hydrogenolysis, oxidation, hydrogenation, coupling reactions etc. as well as for CO2 capture. We have also developed a new method of fabricating active photocatalysts by TiO2 coating of DFNS and plasmonic black gold.
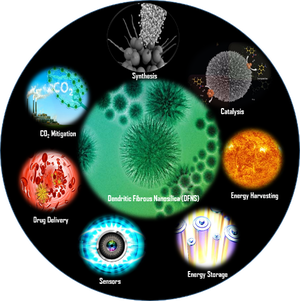
In the nano-catalysis (NanoCat) laboratory, we are designing and synthesizing various nano-materials (silica, metal oxides, metals, etc) with specific shapes, sizes and morphologies and then evaluating their use as a nano-catalysts for the development of sustainable protocols for various processes like photocatalysis, CO2 capture and conversion to fine chemicals, environmental remediation as well as C-H activation, C-C coupling, oxidation, metathesis, hydrogenolysis, hydrogenation reactions.
A guiding hypothesis is that catalytic efficiency (activity, kinetics, selectivity and stability) can be controlled by tuning the morphology of nanomaterials/nanocatalysts.
In the nano-catalysis (NanoCat) laboratory, we are designing and synthesizing various nano-materials (silica, metal oxides, metals, etc) with specific shapes, sizes and morphologies and then evaluating their use as a nano-catalysts for the development of sustainable protocols for various processes like photocatalysis, CO2 capture and conversion to fine chemicals, environmental remediation as well as C-H activation, C-C coupling, oxidation, metathesis, hydrogenolysis, hydrogenation reactions.
A guiding hypothesis is that catalytic efficiency (activity, kinetics, selectivity and stability) can be controlled by tuning the morphology of nanomaterials/nanocatalysts.
Highlights of Prof. Polshettiwar’s group’s research are summarized below,
1) Nanosponges of Acidic Amorphous Aluminosilicate for Catalysis, Plastic Degradation and CO2 to Fuel Conversion
In this work, we report the synthesis and application of a different class of material, called “Acidic Amorphous Aluminosilicates (AAS)”, which possesses Brønsted acidic sites like in zeolites and textural properties like ASAs. AAS catalyzes eight different reactions (styrene oxide ring-opening, vesidryl synthesis, Friedel−Crafts alkylation, jasminaldehyde synthesis, m-Xylene isomerization and cumene cracking) which all require strong acidic sites and larger pore sizes, with better performance than state-of-the-art zeolites and amorphous aluminosilicates. Notably, AAS efficiently converts a range of waste plastics to hydrocarbons at significantly lower temperatures. A Cu-Zn-Al/AAS hybrid shows excellent performance for CO2 to fuel conversion with 79% selectivity for dimethyl ether. The catalytic performance of AAS is then investigated by conventional and DNP-enhanced solid-state NMR to provide molecular-level understanding of the distinctive Brønsted acidic sites of these materials. Due to their unique combination of strong acidity and accessibility, AAS will be a potential alternative to hierarchical zeolites.
Ref. Nature Communications, 2020 in press.
In this work, we report the synthesis and application of a different class of material, called “Acidic Amorphous Aluminosilicates (AAS)”, which possesses Brønsted acidic sites like in zeolites and textural properties like ASAs. AAS catalyzes eight different reactions (styrene oxide ring-opening, vesidryl synthesis, Friedel−Crafts alkylation, jasminaldehyde synthesis, m-Xylene isomerization and cumene cracking) which all require strong acidic sites and larger pore sizes, with better performance than state-of-the-art zeolites and amorphous aluminosilicates. Notably, AAS efficiently converts a range of waste plastics to hydrocarbons at significantly lower temperatures. A Cu-Zn-Al/AAS hybrid shows excellent performance for CO2 to fuel conversion with 79% selectivity for dimethyl ether. The catalytic performance of AAS is then investigated by conventional and DNP-enhanced solid-state NMR to provide molecular-level understanding of the distinctive Brønsted acidic sites of these materials. Due to their unique combination of strong acidity and accessibility, AAS will be a potential alternative to hierarchical zeolites.
Ref. Nature Communications, 2020 in press.
2) Dendritic Fibrous Nanosilica: All-in-one Nanomaterial for Energy, Environment and Health
We have developed next-generation nanocatalysts via the morphological control of nanomaterials, particularly dendritic fibrous nanosilica (DFNS). His group has shown the successful utilization of DFNS in a range of important applications, including catalysis and CO2 capture/conversion to fuel. DFNS is one of the few Indian invented material, which is now being explored by more than 150 research groups worldwide for various applications. Dendritic fibrous nanosilica (DFNS) attracted a great deal of attention in a large number of scientific disciplines such as catalysis, solar energy harvesting (photocatalysis, solar cells, etc.), energy storage, self-cleaning antireflective coatings, surface plasmon resonance (SPR)-based ultra-sensitive sensors, CO2 capture, and biomedical applications (drug delivery, protein and gene delivery, bioimaging, photothermal ablation, Ayurvedic and radiotherapeutics drug delivery, etc.)
Ref: Nature Protocol, 2019, 14, 2177-2204 and ChemSusChem 2017, 10, 3866-3913.
We have developed next-generation nanocatalysts via the morphological control of nanomaterials, particularly dendritic fibrous nanosilica (DFNS). His group has shown the successful utilization of DFNS in a range of important applications, including catalysis and CO2 capture/conversion to fuel. DFNS is one of the few Indian invented material, which is now being explored by more than 150 research groups worldwide for various applications. Dendritic fibrous nanosilica (DFNS) attracted a great deal of attention in a large number of scientific disciplines such as catalysis, solar energy harvesting (photocatalysis, solar cells, etc.), energy storage, self-cleaning antireflective coatings, surface plasmon resonance (SPR)-based ultra-sensitive sensors, CO2 capture, and biomedical applications (drug delivery, protein and gene delivery, bioimaging, photothermal ablation, Ayurvedic and radiotherapeutics drug delivery, etc.)
Ref: Nature Protocol, 2019, 14, 2177-2204 and ChemSusChem 2017, 10, 3866-3913.
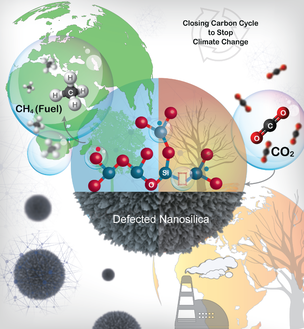
3) Closing the Carbon Cycle to Stop Climate Change: The First Metal-Free-Ligand-Free Catalyst for CO2 to Fuel Transformation
We have developed the magnesiothermic defect engineering protocol to design a new catalyst system where metal nanoparticle active sites replaced with defects as catalytically active sites. This is the first metal-free-ligand-free catalyst for CO2 conversion. The defects in nanosilica convert CO2 to methane with excellent productivity and selectivity. Furthermore, metal nanoparticles were not required, and the defect sites alone acted as catalytic sites for carbon dioxide activation and hydrogen dissociation and their cooperative action converted CO2 to methane. The catalyst is recyclable and stable for more than 200 h with 10000 µmoles g-1 h-1 of productivity for methane. Notably, unlike expensive metal catalysts, the catalytic activity for methane production increased significantly after every regeneration cycle, reaching more than double the methane production rate after eight regeneration cycles as compared to the initial catalyst performance.
Ref: Proc. Natl. Acad. Sci. U.S.A 2020, 117, 6383-6390.
We have developed the magnesiothermic defect engineering protocol to design a new catalyst system where metal nanoparticle active sites replaced with defects as catalytically active sites. This is the first metal-free-ligand-free catalyst for CO2 conversion. The defects in nanosilica convert CO2 to methane with excellent productivity and selectivity. Furthermore, metal nanoparticles were not required, and the defect sites alone acted as catalytic sites for carbon dioxide activation and hydrogen dissociation and their cooperative action converted CO2 to methane. The catalyst is recyclable and stable for more than 200 h with 10000 µmoles g-1 h-1 of productivity for methane. Notably, unlike expensive metal catalysts, the catalytic activity for methane production increased significantly after every regeneration cycle, reaching more than double the methane production rate after eight regeneration cycles as compared to the initial catalyst performance.
Ref: Proc. Natl. Acad. Sci. U.S.A 2020, 117, 6383-6390.

4) Black (nano)Gold Combat Climate Change
We have developed the solution-phase synthesis of Dendritic Plasmonic Colloidosomes (DPCs, a black gold) with varying interparticle distances between the gold Nanoparticles (NPs) using a cycle-by-cycle growth approach by optimizing the nucleation-growth step. These DPCs absorbed the entire visible and near-infrared region of solar light, due to interparticle plasmonic coupling as well as the heterogeneity in the Au NP sizes, which transformed golden gold material to black gold. Black (nano) gold was able to catalyze CO2 to methane (fuel) conversion at atmospheric pressure and temperature, using solar energy. Similar to the real trees, the developed black gold acts like an artificial tree that uses CO2, sunlight and water to produce fuel, which can be used to run our cars. Notably, black gold can
also be used to convert seawater into drinkable water using the heat that
black gold generates after it captures sunlight.
Ref: Chemical Science, 2019, 10, 6694-6603.
We have developed the solution-phase synthesis of Dendritic Plasmonic Colloidosomes (DPCs, a black gold) with varying interparticle distances between the gold Nanoparticles (NPs) using a cycle-by-cycle growth approach by optimizing the nucleation-growth step. These DPCs absorbed the entire visible and near-infrared region of solar light, due to interparticle plasmonic coupling as well as the heterogeneity in the Au NP sizes, which transformed golden gold material to black gold. Black (nano) gold was able to catalyze CO2 to methane (fuel) conversion at atmospheric pressure and temperature, using solar energy. Similar to the real trees, the developed black gold acts like an artificial tree that uses CO2, sunlight and water to produce fuel, which can be used to run our cars. Notably, black gold can
also be used to convert seawater into drinkable water using the heat that
black gold generates after it captures sunlight.
Ref: Chemical Science, 2019, 10, 6694-6603.
5) Highly Monodisperse Dendritic Fibrous Nanosilica: Scalable Synthesis Quantified by E-Factor
Successful use of DFNS hinges critically on the development of scalable synthesis methods to provide technologically significant quantities of high-quality nanomaterials with tunable size, morphology, and textural properties. This study details a simple, versatile, scalable synthesis route for DFNS. We develop a DFNS synthesis process by replacing the microwave-assisted close-reactor synthesis protocol with a round bottom flask-based open reactor protocol. We found an exceptional E-factor value of 15 for the DFNS protocol, which is several orders of magnitude better than other reported processes.
(Ref- ACS Appl. Nanomat., 2018, 1, 3636; ACS Appl. Mater. Interfaces, 2018, 10, 23392.
Successful use of DFNS hinges critically on the development of scalable synthesis methods to provide technologically significant quantities of high-quality nanomaterials with tunable size, morphology, and textural properties. This study details a simple, versatile, scalable synthesis route for DFNS. We develop a DFNS synthesis process by replacing the microwave-assisted close-reactor synthesis protocol with a round bottom flask-based open reactor protocol. We found an exceptional E-factor value of 15 for the DFNS protocol, which is several orders of magnitude better than other reported processes.
(Ref- ACS Appl. Nanomat., 2018, 1, 3636; ACS Appl. Mater. Interfaces, 2018, 10, 23392.
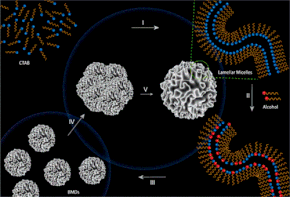
6) Unraveling the Formation Mechanism of Dendritic Fibrous Nanosilica
We have demonstrated the use of coalescence of BMDs to establish the precise formation mechanism of nanomaterials, for DFNS. It followed self-assembly of CTBA and alcohols to form lamellar phases, which then formed bicontinuous microemulsion droplets. These BMDs underwent coalescence to from nano-reactor (a template), within which nucleation and growth steps took place to yield silica nanospheres with fibrous morphology. We understood the mechanism by using theoretical calculations, cryo-TEM, and DLS studies.
(Ref- Langmuir, 2017, 33, 13774)
We have demonstrated the use of coalescence of BMDs to establish the precise formation mechanism of nanomaterials, for DFNS. It followed self-assembly of CTBA and alcohols to form lamellar phases, which then formed bicontinuous microemulsion droplets. These BMDs underwent coalescence to from nano-reactor (a template), within which nucleation and growth steps took place to yield silica nanospheres with fibrous morphology. We understood the mechanism by using theoretical calculations, cryo-TEM, and DLS studies.
(Ref- Langmuir, 2017, 33, 13774)
7) Turning Soft Template into Hard Template: Solution-Phase Synthesis of Tunable Nanosheets of Silica and Carbon
Solution-phase synthesis of silica nanosheets with tunable thickness and textural properties is still a challenge. We have developed a simple and robust protocol to synthesize silica nanosheets using lamellar micelles as soft templates in water-cyclohexane solvents mixture.
(Ref- Nanoscale, 2019, 11, 5365 and Chemistry Select, 2018, 3, 10684)
Solution-phase synthesis of silica nanosheets with tunable thickness and textural properties is still a challenge. We have developed a simple and robust protocol to synthesize silica nanosheets using lamellar micelles as soft templates in water-cyclohexane solvents mixture.
(Ref- Nanoscale, 2019, 11, 5365 and Chemistry Select, 2018, 3, 10684)
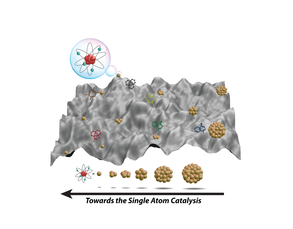
8) Fibrous Nanosilica Supported Ultrasmall Nanoparticles and Pseudo-Single Atoms of Gold and Platinum as Catalysts and Artificial Enzyme
The combination of ultrasmall nanoparticles and pseudo-single atoms of gold (Au) and DFNS functionalized with APTS enabled the design of nanocatalysts with very high turnover numbers (TONs). DFNS/Au catalysed the oxidation of organosilanes to silanols, with a TON of approximately a half million. Also, we observed that in DFNS/Pt catalysts with sub-nanometer Pt or pseudo-single atoms of Pt had excellent selectivity. Thus, while most catalysts offer good activity or selectivity, these DFNS based catalysts offer both high activity and selectivity. We even found that DFNS supported gold (Au) nanoparticles (DFNS/Au) as peroxidase-like artificial enzyme.
(Ref- J. Mat. Chem. A. 2017, 5, 1935; J. Mat. Chem. A. 2016, 4, 12416; J. Mat. Chem. B. 2018, 1600)
The combination of ultrasmall nanoparticles and pseudo-single atoms of gold (Au) and DFNS functionalized with APTS enabled the design of nanocatalysts with very high turnover numbers (TONs). DFNS/Au catalysed the oxidation of organosilanes to silanols, with a TON of approximately a half million. Also, we observed that in DFNS/Pt catalysts with sub-nanometer Pt or pseudo-single atoms of Pt had excellent selectivity. Thus, while most catalysts offer good activity or selectivity, these DFNS based catalysts offer both high activity and selectivity. We even found that DFNS supported gold (Au) nanoparticles (DFNS/Au) as peroxidase-like artificial enzyme.
(Ref- J. Mat. Chem. A. 2017, 5, 1935; J. Mat. Chem. A. 2016, 4, 12416; J. Mat. Chem. B. 2018, 1600)
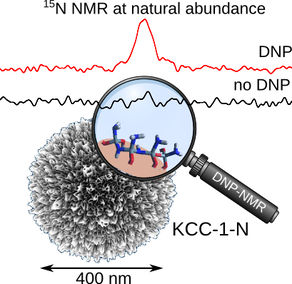
9) Silica-Oxynitrides as Solid Base and Unavailing their Catalytic Sites by using NMR and Dynamic Nuclear Polarization (DNP) Enhanced Solid-State NMR
Silica-oxynitrides are promising solid base catalysts and CO2 sorbents for various industries. Interestingly, higher nitridation temperatures increase the nitrogen content of these silicon oxynitrides of DFNS, but, paradoxically, decreases their catalytic activity. This property was not explained earlier and in this work, we were able to explain this fundamental phenomenon using dynamic nuclear polarization (DNP) enhanced solid-state NMR and we could also propose a detailed mechanism of nitridation reaction.
(Ref- Angew. Chem. Int. Ed. 2015, 54, 2190 ; Angew. Chem. Int. Ed. 2015, 54, 5985).
Silica-oxynitrides are promising solid base catalysts and CO2 sorbents for various industries. Interestingly, higher nitridation temperatures increase the nitrogen content of these silicon oxynitrides of DFNS, but, paradoxically, decreases their catalytic activity. This property was not explained earlier and in this work, we were able to explain this fundamental phenomenon using dynamic nuclear polarization (DNP) enhanced solid-state NMR and we could also propose a detailed mechanism of nitridation reaction.
(Ref- Angew. Chem. Int. Ed. 2015, 54, 2190 ; Angew. Chem. Int. Ed. 2015, 54, 5985).
10) Active and Leach Proof Supported Metal Nanocatalysts
We have developed a simple and sustainable protocol for the synthesis of monodisperse metal nanoparticles of a range of metals, supported on DFNS. The use of expensive dendrimer was replaced by inexpensive polyethylenimine to produce highly monodispersed supported metal nanocatalysts.
(Ref- ACS Sustain. Chem. Eng., 2015, 5, 3224; Green Chem. 2016, 18, 5809; ChemPlusChem 2016, 81, 1142)
We have developed a simple and sustainable protocol for the synthesis of monodisperse metal nanoparticles of a range of metals, supported on DFNS. The use of expensive dendrimer was replaced by inexpensive polyethylenimine to produce highly monodispersed supported metal nanocatalysts.
(Ref- ACS Sustain. Chem. Eng., 2015, 5, 3224; Green Chem. 2016, 18, 5809; ChemPlusChem 2016, 81, 1142)
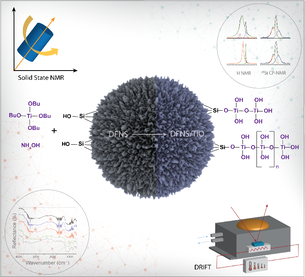
11) DFNS Coated TiO2 as Highly Active Photocatalysts
We have synthesized high surface area photocatalysts by coating TiO2 on the fibrous nano-silica (DFNS) using atomic layer deposition (ALD). Our developed catalyst showed enhanced photo-catalytic activity, better than well-known MCM-41 and SBA-15 supported TiO2 catalysts. Then, sustainable solution-phase TiO2 deposition on silica protocol was developed over complex and expensive ALD techniques. We then further developed perfect crystallization of active sites in supported TiO2 photocatalysts, by a unique combination of nanomaterial morphology and active site crystallization protocol. This yielded nano-TiO2 based photo-catalysts with one of the highest reported photocatalytic hydrogen yields.
(Ref- ACS Catalysis 2016, 6, 2770; ChemSusChem, 2017, 10, 2182; ChemPhotoChem 2018, 2, 796; ChemNanoMat, 2018, 4, 1231)
We have synthesized high surface area photocatalysts by coating TiO2 on the fibrous nano-silica (DFNS) using atomic layer deposition (ALD). Our developed catalyst showed enhanced photo-catalytic activity, better than well-known MCM-41 and SBA-15 supported TiO2 catalysts. Then, sustainable solution-phase TiO2 deposition on silica protocol was developed over complex and expensive ALD techniques. We then further developed perfect crystallization of active sites in supported TiO2 photocatalysts, by a unique combination of nanomaterial morphology and active site crystallization protocol. This yielded nano-TiO2 based photo-catalysts with one of the highest reported photocatalytic hydrogen yields.
(Ref- ACS Catalysis 2016, 6, 2770; ChemSusChem, 2017, 10, 2182; ChemPhotoChem 2018, 2, 796; ChemNanoMat, 2018, 4, 1231)

12) Design of CO2 Sorbents using Functionalized Fibrous Nanosilica and Insights into the Effect of Silica Morphology on its Efficiency
Hybrid materials by functionalization of fibrous nano-silica were synthesized for efficient CO2 capture. Functionalization was achieved by simple physisorption and covalent attachment of various amine molecules. DFNS-TEPAads was compared with its MCM-41 counterpart (MCM-41/TEPAads) and was found far better in terms of CO2 capture capacity, the rate of adsorption and stability.
(Ref- Chem. Sci., 2012, 3, 4222; J. Mat. Chem. A. 2016, 4, 7005 )
Hybrid materials by functionalization of fibrous nano-silica were synthesized for efficient CO2 capture. Functionalization was achieved by simple physisorption and covalent attachment of various amine molecules. DFNS-TEPAads was compared with its MCM-41 counterpart (MCM-41/TEPAads) and was found far better in terms of CO2 capture capacity, the rate of adsorption and stability.
(Ref- Chem. Sci., 2012, 3, 4222; J. Mat. Chem. A. 2016, 4, 7005 )