Green & Sustainable Chemistry
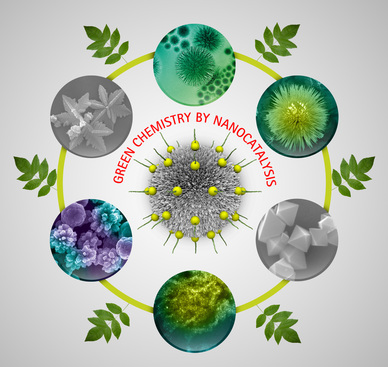
Catalysis, which provides sustainable, economical and efficient ways to convert raw materials into valuable chemicals and fuels, is essential to the development of modern society. With the aim to avoid the use of volatile organic solvents, toxic reagents, hazardous and/or harsh reaction conditions as well as challenging and time-consuming wasteful separations, greener and environmentally benign catalytic protocols have recently become more popular. In this regard, the development of nanoscience, undreamt of a century ago, has made the greening of chemistry possible.
Ref: 1. Green Chemistry by Nano-catalysis. Vivek Polshettiwar* and R. S. Varma, Green Chem. 2010, 12, 743-754.
2. Nanoscience Makes Catalysis Greener. Vivek Polshettiwar,* Jean-Marie Basset,* and Didier Astruc*, ChemSusChem, 2012, 5, 6-8.
2. Nanoscience Makes Catalysis Greener. Vivek Polshettiwar,* Jean-Marie Basset,* and Didier Astruc*, ChemSusChem, 2012, 5, 6-8.
Magnetically Recoverable Nano-Catalysts
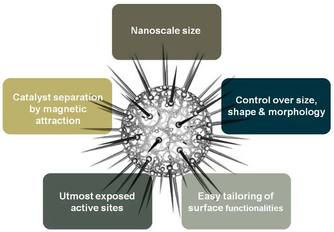
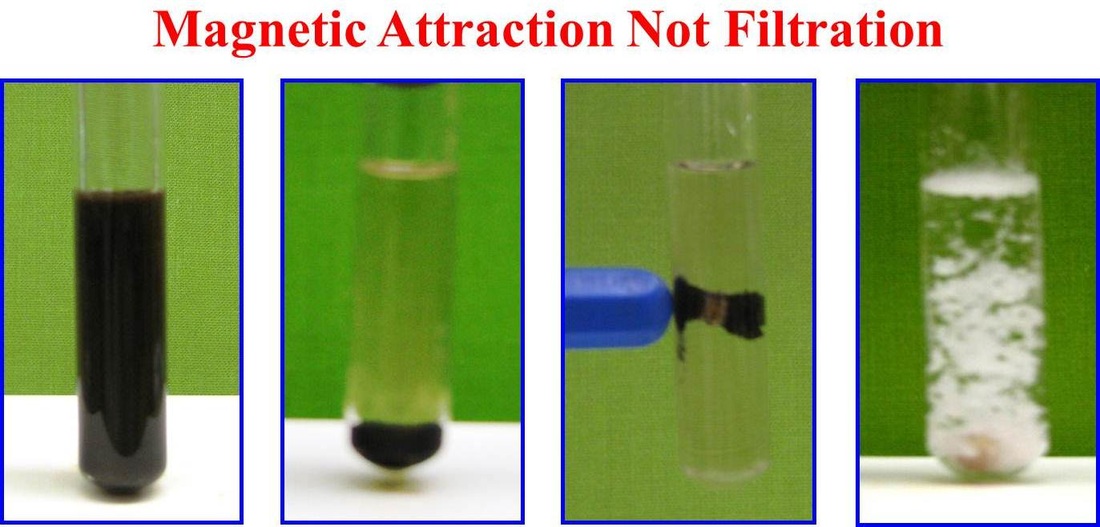
Although nanocatalysts enjoy several advantages over conventional catalyst systems; however, isolation and recovery of these tiny nanocatalysts from the reaction mixture is not easy. Conventional techniques (such as filtration) are not efficient because of the nano size of the catalyst particles. This limitation hampers the economics and sustainability of these nanocatalytic protocols. To overcome this issue, the use of magnetic nanoparticles has emerged as a viable solution; their insoluble and paramagnetic nature enables easy and efficient separation of the catalysts from the reaction mixture with an external magnet. We envision designing and preparing magnetically separable nano-catalysts that can be utilized for future challenges on the horizon. We believe, it is only a matter of time before the real prospective of magnetic nanocatalysts are realized and implemented on a far-reaching scale.
Although nanocatalysts enjoy several advantages over conventional catalyst systems; however, isolation and recovery of these tiny nanocatalysts from the reaction mixture is not easy. Conventional techniques (such as filtration) are not efficient because of the nano size of the catalyst particles. This limitation hampers the economics and sustainability of these nanocatalytic protocols. To overcome this issue, the use of magnetic nanoparticles has emerged as a viable solution; their insoluble and paramagnetic nature enables easy and efficient separation of the catalysts from the reaction mixture with an external magnet. We envision designing and preparing magnetically separable nano-catalysts that can be utilized for future challenges on the horizon. We believe, it is only a matter of time before the real prospective of magnetic nanocatalysts are realized and implemented on a far-reaching scale.
Ref: 1. Magnetically Recoverable Nano-Catalysts. V. Polshettiwar,* R. Luque, A. Fihri, H. Zhu, J. M. Basset, Chem. Rev. 2011, 111, 3036.
2. Vivek Polshettiwar* and R. S. Varma, Chem. Eur. J. 2009, 15, 1582-1586.
3. Vivek Polshettiwar,* Babita Baruwati, and R. S. Varma, Chem.Commun. 2009, 1837-1839.
4. Vivek Polshettiwar* and R. S. Varma, Green Chem. 2009, 11, 1313-1316..
.
2. Vivek Polshettiwar* and R. S. Varma, Chem. Eur. J. 2009, 15, 1582-1586.
3. Vivek Polshettiwar,* Babita Baruwati, and R. S. Varma, Chem.Commun. 2009, 1837-1839.
4. Vivek Polshettiwar* and R. S. Varma, Green Chem. 2009, 11, 1313-1316..
.
Aqueous MW Chemistry
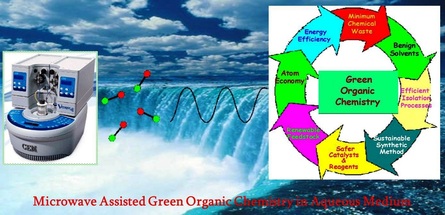
Catalyzed organic transformations in aqueous reaction medium are one of the ideal solutions for the development of green and sustainable protocols. However, execution of many organic reactions in water is not simple due to the inherent limitation of solubility of non-polar reactants in polar aqueous medium, which can be overcome by using MW irradiation conditions. Thus, the fusion of benign water medium, non-conventional MW heating, and nano-catalyst seems to be the preeminent way to develop the next generation of highly efficient processes.
Catalyzed organic transformations in aqueous reaction medium are one of the ideal solutions for the development of green and sustainable protocols. However, execution of many organic reactions in water is not simple due to the inherent limitation of solubility of non-polar reactants in polar aqueous medium, which can be overcome by using MW irradiation conditions. Thus, the fusion of benign water medium, non-conventional MW heating, and nano-catalyst seems to be the preeminent way to develop the next generation of highly efficient processes.
Ref: 1. Suzuki-Miyaura cross-coupling reactions in aqueous media: Sustainable syntheses of biaryls.
Vivek Polshettiwar,* Audrey Decottignies, Christophe Len and Aziz Fihri, Chem. Sus. Chem. 2010, 3, 502-522.
2. Silica-supported palladium catalysts for cross-coupling reactions: syntheses and catalysis.
Vivek Polshettiwar,* Christophe Len and Aziz Fihri, Coord. Chem. Rev. 2009, 253, 2599-2626.
3. Aqueous microwave chemistry: a clean and green synthetic tool for rapid drug discovery.
Vivek Polshettiwar* and R. S. Varma, Chem. Soc. Rev. 2008, 37, 1546-1557.
4. Microwave-assisted organic synthesis and transformations using benign reaction media.
Vivek Polshettiwar and R. S. Varma, Acc. Chem. Res. 2008, 41, 629-639
Vivek Polshettiwar,* Audrey Decottignies, Christophe Len and Aziz Fihri, Chem. Sus. Chem. 2010, 3, 502-522.
2. Silica-supported palladium catalysts for cross-coupling reactions: syntheses and catalysis.
Vivek Polshettiwar,* Christophe Len and Aziz Fihri, Coord. Chem. Rev. 2009, 253, 2599-2626.
3. Aqueous microwave chemistry: a clean and green synthetic tool for rapid drug discovery.
Vivek Polshettiwar* and R. S. Varma, Chem. Soc. Rev. 2008, 37, 1546-1557.
4. Microwave-assisted organic synthesis and transformations using benign reaction media.
Vivek Polshettiwar and R. S. Varma, Acc. Chem. Res. 2008, 41, 629-639